|
|
|
1.
|
A chemical reaction is most likely to be spontaneous if it is accompanied
by
A) | increasing energy and increasing entropy | B) | lowering energy and
increasing entropy | C) | increasing energy and decreasing
entropy | D) | lowering energy and decreasing entropy | E) | none of these
(A-D) |
|
|
|
2.
|
Which of the following is true for exothermic processes?
A) | DSsurr < 0 | B) | DSsurr = –DH/T | C) | DSsurr = 0 | D) | DSsurr > 0 | E) | two of
these |
|
|
|
3.
|
As long as the disorder of the surroundings is increasing, a process will be
spontaneous.
|
|
|
4.
|
For any given process, DSsurr and
DSsys have opposite signs.
|
|
|
5.
|
Substance X has a heat of vaporization of 64.4 kJ/mol at its normal boiling
point (423°C). For the process X(l) ® X(g) at 1
atm and 423°C calculate the value of DG.
A) | 0 J | B) | 92.5 J | C) | 152
J | D) | –92.5 J | E) | –152 J |
|
|
|
6.
|
DH° is zero for a chemical reaction at
constant temperature.
|
|
|
7.
|
Which item (a, b or c) in each of the three groups below has the lowest
entropy?
I. (a) 10 g ice (b) 10 g
water vapor (c) 10 g liquid water
II.
(a) 1 mole NaCl solid (b) 1 mol NaCl in 1 M aqueous solution (c) 1 mol
molten NaCl
III. (a) 1 mole C2H6(g), (b) 1
mole CH4(g), (c) 1 mol C3H8(g) all at 25 °C and 1 atm
A) | a, a, b | B) | c, b, c | C) | b, b,
c | D) | a, b, c | E) | a, a, c |
|
|
|
8.
|
Which of the following statements is true concerning the electrochemical cell
depicted below?
Ca | Ca2+(aq) || K+(aq) |
K
Ca2+(aq) + 2e– ®
Ca(s); e° = –2.87 V
K+(aq) +
e– ® K(s); e° = –2.93 V
A) | The cell reaction is spontaneous with a standard cell potential of 0.06
V. | B) | The cell reaction is nonspontaneous with a standard cell potential of –5.80
V. | C) | The cell reaction is nonspontaneous with a standard cell potential of –0.06
V. | D) | The cell reaction is spontaneous with a standard cell potential of 5.80
V. | E) | The cell is at equilibrium. |
|
|
|
9.
|
In which of the following cases must e be
equal to zero?
A) | In any cell at equilibrium. | B) | In a concentration cell. | C) | e can never be equal to zero. | D) | Choices A and B are both
correct. | E) | None of these. |
|
|
|
10.
|
Which of the following statements is true about a voltaic cell for which
e°cell = 1.00 V?
A) | It has DG° > 0. | B) | The system is at
equilibrium. | C) | It has K = 1. | D) | The cathode is at a higher energy than the
anode. | E) | The reaction is spontaneous. |
|
|
|
11.
|
Concentration cells work because standard reduction potentials are dependent on
concentration.
|
|
|
12.
|
Which of the following statements about batteries is false?
A) | A battery is a group of galvanic cells connected in series. | B) | Lead storage
batteries contain lead at the anode and lead coated with lead dioxide at the
cathode. | C) | The alkaline dry cell battery can last longer than a nickel-cadmium
battery. | D) | A fuel cell is a galvanic cell for which the reactants are continuously
supplied. | E) | Dry cell batteries are used in tape players and portable
radios. |
|
|
|
13.
|
Balance the following equation: Cr2O72– +
I– ® Cr3+ +
IO3– (acid)
|
|
|
14.
|
Balance the following equation: Bi(OH)3 +
SnO22– ® Bi +
SnO32– (base)
|
|
|
15.
|
The so-called “magic numbers” of protons and neutrons produce
special chemical stability.
|
|
|
16.
|
Which statement is true about the following reaction? | | | 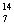 N | + | 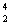 He | ® | 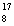 O | + | 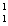 H | | | | 13.992 | | 4.0015 | | 16.9986 | | 1.0073 | | | | | amu | | amu | | amu | | amu | | | | | | | | | | |
A) | Energy is absorbed in the reaction. | B) | Energy is released in the
reaction. | C) | No energy change is associated with the reaction. | D) | Not enough
information is given to determine the energy change. | E) | None of these. |
|
|
|
17.
|
Which of the following is true for the fission of uranium-235?
A) | The electron is captured by the nucleus, which becomes unstable. | B) | The products include
neutrons. | C) | The nuclides produced are individually heavier than the uranium
nuclide. | D) | The nuclides produced are more stable than the uranium nuclide. | E) | Two of
these. |
|
|
|
18.
|
Which of the following statements (A-D) is false?
A) | The process of splitting a heavy nucleus into two nuclei with smaller mass numbers is
called fission. | B) | A beta particle is a particle with the same mass as the electron but opposite
charge. | C) | Nitrogen can be changed into oxygen by bombarding it with alpha
particles. | D) | Archaeologists use radioactivity to determine the age of some artifacts and
rocks. | E) | All of the above statements are true. |
|
|
|
19.
|
Which of the following is the second most abundant (by mass) element in the
earth's crust, oceans, and atmosphere?
A) | hydrogen | B) | oxygen | C) | carbon | D) | aluminum | E) | silicon |
|
|
|
20.
|
Because Li is the strongest reducing agent of the alkali metals, it reacts most
quickly with water of the alkali metals.
|
|
|
21.
|
Alkaline earth metals react less vigorously with water than do the alkali
metals.
|
|
|
22.
|
The Group 3A elements are all metals.
|
|
|
23.
|
Choose the correct molecular structure for PCl3.
A) | trigonal bipyramidal | B) | trigonal planar | C) | tetrahedral | D) | octahedral | E) | none of
these |
|
|
|
24.
|
Pi bonding tends to be important in all elements of Group 5A.
|
|
|
25.
|
Which of the following is not a use of hydrazine?
A) | Blowing agent. | B) | Fungicides. | C) | Herbicides. | D) | Insecticides. | E) | All are uses of
hydrazine. |
|
|
|
26.
|
Choose the species with the largest ionization energy.
A) | F | B) | Cl | C) | Br | D) | I | E) | All are the
same. |
|
|
|
27.
|
The bond strength of HCl is greater than that of HF.
|
|
|
28.
|
The acid HClO4 is a stronger acid than HClO2.
|
|
|
Write a balanced equation for each of the following reactions:
|
|
|
29.
|
Lithium metal with H2O(l).
|
|
|
Write a balanced equation for each of the following:
|
|
|
30.
|
The oxidation of copper metal by 6 M nitric acid
|
|
|
31.
|
In which of the following compounds does N have its maximum oxidation
state?
A) | NH3 | B) | N2O | C) | N2 | D) | NaNO3 | E) | HN3 |
|
|
|
32.
|
Which of the following transition metals is most likely to form an oxide?
A) | gold | B) | silver | C) | platinum | D) | palladium | E) | copper |
|
|
|
33.
|
The metals with the highest ionization energies are most likely to be found in
nature in the elemental state.
|
|
|
34.
|
Metals usually have higher melting points than nonmetals.
|
|
|
35.
|
The expected electron configuration of Cu+ is [Ar]
3s13d9.
|
|
|
36.
|
The electron configuration of Mn2+ is [Ar]
4s23d3.
|
|
|
37.
|
Because they have the same atoms, bonds, and formulas, geometrical isomers have
the same color.
|
|
|
38.
|
All tetrahedral complex ions are high spin.
|
|
|
39.
|
The complex ions containing Zn2+ are intensely colored.
|
|
|
40.
|
The complexes of Zn2+ are all diamagnetic.
|
|
|
41.
|
Define stereoisomerism.
|
|
|
How many unpaired electrons are found in each of the following complex
ions?
|
|
|
42.
|
NiCl42–
|
|
|
43.
|
FeCl42–
|
|
|
44.
|
[Cr(CN)4]2–
|
|
|
45.
|
Which of the following names is a correct one?
A) | 3-methyl-4-isopropylpentane | B) | 2-ethyl-4-tertiary-butylpentane | C) | 2,2,3,5-tetramethylheptane | D) | t-butylethane | E) | trans-1,2-dimethylethane |
|
|
|
46.
|
Cyclobutane has 109° bond angles like all alkanes.
|
|
|
47.
|
Which of the following is known as rubbing alcohol?
A) | methanol | B) | ethanol | C) | propanol | D) | isopropanol | E) | none of
these |
|
|
|
48.
|
Which of the following has an optical isomer?
|
|
|
49.
|
The structure of the polymer used in a freezer wrap can mainly be described as
follows:
[CCl2 –CH2 –CCl2–CH2
–CCl2 –CH2 –CCl2
–CH2]n
The chief monomer of this wrap would have which
structure?
A) | CCl2=CH2 | B) | Cl2C–CH2 | C) | Cl2C=CH2=CCl2 | D) | CCl2 | E) | none of these |
|
|
|
50.
|
Which of the following polymers is not based on a substituted ethylene
monomer?
A) | nylon | B) | polyvinylchloride | C) | Teflon | D) | polystyrene | E) | polypropylene |
|